BIOLOGICAL NUTRIENT REMOVAL
Glen T. Daigger
Vice-President, CH2M-HILL
Denver, Colorado
INTRODUCTION
The environmental protection profession is increasingly recognizing the adverse impacts that can be caused by the discharge of the nutrients nitrogen and phosphorus to the aquatic environment (National Research Council, 1993). Ammonia-nitrogen discharges can lead to increased oxygen demand in the receiving stream and depressed dissolved oxygen concentrations. Ammonia-nitrogen can be toxic to aquatic organisms, depending on the pH and temperature conditions within the receiving water body. Nitrite-nitrogen can also be quite toxic. Nitrogen and phosphorus discharges can enrich receiving water bodies, resulting in the undesirable growth of algae and other aquatic plants which is known as eutrophication. Nitrate-nitrogen discharges can also be a public health concern due to the 10 mg-N/L primary drinking water standard. Table 1 summarizes the impacts of nutrient discharges on receiving waters.
As a consequence, nutrient limitations are increasingly being added to the discharge permits of wastewater treatment plants. Chemical systems are used frequently to remove phosphorus. However, interest and experience is increasing rapidly in the use of biological systems to remove both nitrogen and phosphorus. These systems are commonly referred to as biological nutrient removal (BNR) systems. This paper will: (1) discuss the mechanisms which provide nitrogen and phosphorus removal in BNR systems, (2) summarize performance information and relationships, and (3) describe resources available to wastewater treatment professionals who must consider nutrient removal options.
TABLE 1
Impact of Nutrient Discharges
Nutrient Impact
Ammonia Oxygen Demand, Toxicity
Nitrite Toxicity
Nitrate Water Supply Impacts
Total Nitrogen Eutrophication
Total Phosphorus Eutrophication
NUTRIENT REMOVAL MECHANISMS
A large number of biological nutrient removal process options have been developed and are used in full-scale wastewater treatment plants (Sedlak, 1991). Evaluation of the applicability of this wide variety of process options by potential users can be confusing, particularly for those who are not intimately familiar with the relatively minor differences which can exist between seemingly similar process options. Differences between nutrient removal process options can sometimes result in significant differences in process performance and/or operational characteristics. In other cases, they will make little or no impact. Different process options may also simply represent different approaches to accomplish the same objectives. How can the practitioner distinguish between the various process options available and select the option that best meets the objectives for a particular application? The answer is that the practitioner must understand the mechanisms by which nutrients are removed in biological nutrient removal processes. An understanding of the underlying mechanisms allows the experienced practitioner to identify what factors are significant in determining process performance and, consequently, to determine reasonable performance differences between alternative process options.
This section provides a summary of the mechanisms which operate in BNR systems. The mechanisms operating in biological nitrogen removal systems are discussed first, allowed by the mechanisms operating in biological phosphorus removal systems.
Nitrogen Removal
A certain degree of nitrogen removal occurs in any biological wastewater treatment system due to the uptake of nitrogen into the waste sludge produced in the process. Nitrogen is a component of waste biomass produced as a result of biological treatment of carbonaceous organic matter. Organic nitrogen is also a component of the non-biodegradable particulate organic matter which is present in many wastewaters. This material will generally be flocculated and incorporated into the biological treatment system mixed liquor and subsequently removed from the process with the waste sludge. Standard procedures are available to determine the quantity of nitrogen which will be removed by these mechanisms (Grady and Lim, 1980; Sedlak, 1991). Nitrogen removal will occur by this mechanism in BNR systems, just as it occurs in any biological wastewater treatment system. The difference between a typical biological wastewater treatment system and a BNR system is that, in a BNR system, additional nitrogen removal is achieved by the combined action of the two biological reactions: (1) nitrification and (2) denitrification.
Nitrification
Nitrification is the biological conversion of ammonia-nitrogen to nitrate-nitrogen. It is accomplished by members of a group of bacteria called autotrophs. Autotrophic micro-organisms oxidize inorganic constituents to obtain energy for growth and maintenance, while they obtain carbon for the production of new biomass by the reduction of carbon dioxide. Notice that organic matter is not required for the growth of autotrophic bacteria. Nitrification is actually a two-step reaction. The first step is oxidation of ammonia-nitrogen to nitrite-nitrogen by bacteria of the genus Nitrosomonas. The equation for this reaction, presented in simplified format, is as follows:
NH4 + 1.5 O2 - NO3 + 2H+ + H2O (1)
The second step is the oxidation of nitrite-nitrogen to nitrate-nitrogen by bacteria of the genus Nitrobacter. The simplified equation for this reaction is as follows:
NO2 + 0.5 O2 - NO3 (2)
Under steady-state conditions these two reactions will be in balance and the overall reaction will go essentially to completion. Including the synthesis of new biomass (expressed as the typical composition of biomass), the overall reaction is:
NH4 + 1.83 O2 + 1.98 HCO3 - 0.98 NO3 + 0.021 C5H7NO2 (3)
+ 1.88 H2CO3 + 1.04 H2O
Equation (3) illustrates the stoichiometry of the nitrification reaction. Oxygen is required to oxidize ammonia-nitrogen, and 4.6 mg of O2 is required for each mg of NO3-N generated. Bicarbonate alkalinity is also consumed in the reaction to both neutralize the acid produced (i.e. ammonia-nitrogen is a base while nitrate-nitrogen is an acid) and as required for the synthesis of new biomass (from carbon dioxide which is present as bicarbonate alkalinity). The alkalinity requirement calculated from equation (3) is 7.2 mg of alkalinity as CaCO3 for each mg of NO3-N produced. Biomass yield values are typically low for auotrophic bacteria, and the nitrification reaction is no exception. The yield value for the nitrifiers (both Nitrosomonas and Nitrobacter) is 0.15 mg of bacteria as total suspended solids (TSS) per mg of nitrate-nitrogen generated.
The growth of nitrifying bacteria is affected by a number of factors. First of all, the specific growth rate of the nitrifying bacteria is generally lower than that of the heterotrophic bacteria which oxidize carbonaceous organic matter in biological wastewater treatment systems. The specific growth rate of microorganisms in biological wastewater treatment systems is often characterized as the minimum mean cell residence time (minimum MCRT), which represents the MCRT corresponding to the maximum specific growth rate for the subject mircroorganisms.
When the process is operated at the minimum MCRT, the microorganisms are growing at their maximum rate and are just being washed out of the system. The MCRT must be longer than the minimum MCRT if the organisms are to grow and survive in the system. The MCRT in the portion of the system where nitrification occurs is typically 1.5 to 2.0 times the minimum MCRT.
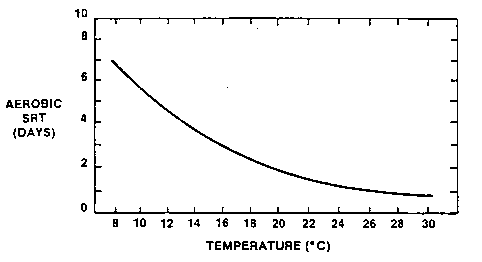
Operation at a MCRT less than the minimum MCRT will result in "washout" of the subject microorganisms. Figure 1 presents the minimum MCRT for the growth of the nitrifying bacteria as a function of temperature and illustrates the temperature sensitivity of the nitrifying bacteria.

The growth of nitrifying bacteria is affected by a number of environmental factors (U.S. EPA, 1993). Some of the most significant ones include dissolved oxygen (DO), pH, and the presence of inhibitors. As illustrated in equation (3), nitrification is an aerobic reaction, that is one that requires the presence of dissolved oxygen. The activity of the nitrifying bacteria is reduced when the dissolved concentration is reduced below about 2 to 3 mg/L, and it is totally inhibited when dissolved oxygen is not being supplied. Nitrification can occur when dissolved oxygen is being supplied by DO concentrations are low, however the rate is reduced.
The activity of the nitrifying bacteria is also affected by pH. The optimum pH for growth of the nitrifying bacteria is generally about 7.5. The activity of the nitrifying bacteria is reduced somewhat as the pH is reduced below 7.0, and it is inhibited significantly when the pH drops below 6.5. Recent information distinguishes between the impact of pH on acclimated and unacclimated cultures (U.S. EPA, 1993). A wide variety of organic and inorganic compounds can also inhibit the growth of the nitrifying bacteria. This can be an important issue if certain industrial wastewaters are being treated (Sedlak, 1991).
Denitrification
Denitrification is the utilization of carbonaceous organic matter by heterotrophic bacteria using nitrate-nitrogen as the terminal electron acceptor (i.e. the "oxygen source"). Many of the heterotrophic bacteria in biological wastewater treatment systems are capable of using either dissolved oxygen or nitrate-nitrogen as a terminal electron acceptor. Dissolved oxygen is used preferentially when both terminal electron acceptors are present. This occurs since slightly more energy can be obtained by the oxidation of carbonaceous organic matter using oxygen as the terminal electron acceptor than when nitrate-nitrogen serves as the terminal electron acceptor. However, dissolved oxygen and nitrate-nitrogen provide essentially the same biochemical function. When nitrate-nitrogen serves as the terminal electron acceptor (i.e. denitrification occurs), the nitrate-nitrogen is converted to nitrogen gas, which can then be liberated into the atmosphere. This reaction in the removal of nitrogen from the wastewater stream.
Denitrification significantly impacts the stoichiometry of a biological wastewater treatment system. For example, the fact that a portion of the carbonaceous oxygen demand is satisfied by the reduction of nitrate-nitrogen means that the process oxygen demands are reduced. Theoretically, 2.86 mg of carbonaceous oxygen demand is satisfied for each mg of NO3-N which is reduced to nitrogen gas.
Denitrification also results in a reduction in process alkalinity consumption due to the removal of the acid nitrate. Theoretically, 3.6 mg of alkalinity as CaCO3 is produced per mg of NO3-N reduced to nitrogen gas.
Table 2 illustrates the complimentary nature of the nitrification and denitrification reactions.
Table 2
Nitrification/Denitrification Process Stoichiometry
Component Nitrification Denitrification
Oxygen 4.6 Consumed 2.86 Demand Satisfied
(mg O2/mg NO3 - N)
Alkalinity 7.2 Consumed 3.60 Produced
(mg as CaCO3/mg NO3 - N)
As illustrated in Figure 2, for municipal wastewaters the denitrification rate varies over a wide range as different fractions of the organic matter contained in the wastewater are oxidized. Initially, a relatively high denitrification rate occurs as the readily biodegradable organic matter is oxidized. When the readily biodegradable organic matter is exhausted, the denitrification rate is reduced to that produced when the more slowly biodegradable organic matter is oxidized. Finally, when all the biodegradable organic matter is oxidized, the denitrification rate is relatively low and is driven only by endogenous respiration. This means that denitrification rates will initially be relatively rapid as the readily biodegradable organic matter is oxidized. The rate will decrease with time, however, as first the readily biodegradable organic matter is depleted and then later as the slowly biodegradable organic matter is depleted. If the organic matter present in the influent wastewater is to be used as the primary carbon source, then it must be used effectively if a relatively high degree of denitrification is to be achieved. This is accomplished by process configurations which use the influent wastewater first for denitrification as discussed below.

Prototype System
Figure 3 illustrates a prototype single sludge biological nitrification/denitrification process. The process illustrated is known as the four-stage Bardenpho process, and it consists of two anoxic zones and two aerobic zones. An anoxic zone is a region of the biological reactor where dissolved oxygen is excluded and nitrate-nitrogen is provided to serve as the terminal electron acceptor. Denitrification occurs in anoxic zones. An aerobic zone is one which is aerated to provide dissolved oxygen as the terminal electron acceptor. When dissolved oxygen is provided in the aerobic zone, both the oxidation of carbonaceous organic matter and nitrification can occur. In the process illustrated in Figure 3, nitrification (the first step in a biological removal  process) occurs in the first aerobic zone.
process) occurs in the first aerobic zone.
Denitrification (the second step in a biological nitrogen removal process) occurs in the firs t and second anoxic zones. Nitrate-nitrogen is provided to the first anoxic zone by the mixed liquor recycle stream from the first aerobic zone to the first anoxic zone. The mixed liquor flowing from the first aerobic zone to the second anoxic zone will contain the nitrate-nitrogen necessary to allow denitrification to occur in the second anoxic zone. The second aerobic zone is relatively small and is used simply to freshen the mixed liquor and physically strip any nitrogen gas bubbles from the mixed liquor before it flows into the clarifier. Table 3 summarizes the functions of the various components of the four-stage Bardenpho process.
Table 3
Function of Bardenpho Process Components
Component Function
First Anoxic Zone High-Rate Denitrification, Driven by Process Influent
Organic Matter
First Aerobic Zone Nitrification
Second Anoxic Zone Low-Rate Denitrification, Drive by Endogenous
Respiration
Second Aerobic Zone Stripping of Nitrogen Gas, Addition of Dissolved Oxygen
To Mixed Liquor
Mixed Liquor Recycle Transport of Nitrate-Nitrogen Generated in the First
Aerobic Zone to the First Anoxic Zone
Several factors affect the performance of a biological nitrogen removal process such as the four-stage Bardenpho process, as illustrated in Table 4. The characteristics of the influent wastewater affect process performance due to the stoichiometric relationship between the quantity of biodegradable organic matter and the nitrogen to be denitrified. As discussed above, theoretically 2.86 mg of carbonaceous oxygen demand must be exerted to denitrify 1 mg of nitrate-nitrogen. Practically, the quantity of carbonaceous organic matter must exceed the theoretical requirement since it is not possible to direct all of the carbonaceous organic matter to denitrification. In general, the 5-day biochemical oxygen demand (BOD5) to total Kjeldahl nitrogen (TKN) ratio (BOD5/TKN) of the influent wastewater must exceed a value of at least four if reasonably complete denitrification is to be achieved. Higher values of this ratio lead to even more complete denitrification. The biodegradability of the wastewater, that is the proportion of the organic matter which is readily biodegradable, also affects the size and performance of the process. If the influent wastewater contains a relatively high proportion of readily biodegradable organic matter, then a high rate of denitrification will result and the performance of the process will be enhanced.
Table 4
Factor Affecting Biological Nitrogen Removal
Wastewater Configuration
* BOD5/TKN * Zone Size and Loading
* Biodegradability * Aeration System Configuration
And Operation
* Temperature * Mixed Liquor Recycle Rate
* Inert Content
* Variability in Characteristics
The sizes of the various zones of the process will affect performance. The first aerobic zone must be sized sufficiently to achieve a relatively high degree of nitrification. The process MCRT calculated based on only the inventory in this zone determines the degree of nitrification which occurs. The MCRT in this zone (known as the aerobic MCRT) must exceed the minimum MCRT for growth of the nitrifiers (as presented in Figure 1) to allow nitrification to occur. As discussed above, generally the actual MCRT in this zone must be 1.5 to 2.0 times the minimum MCRT values presented in Figure 1 if nitrification is to be reliably maintained. Denitrification in the first and second anoxic zones is a function of the zone size and the carbon and nitrogen loadings. Denitrification in the first anoxic zone is a function of: (1) the size of the zone, (2) the mass of nitrate recycled to the zone, and (3) the rate at which the biodegradable organic matter contained in the influent wastewater is biodegraded. The mass of nitrate recycled is a direct function of the mixed liquor recycle flow rates. As illustrated in Figure 4, an increase in the mixed liquor recycle rate increases the mass of nitrate which is recycled to the initial anoxic zone, and the percent nitrate removal which is possible.
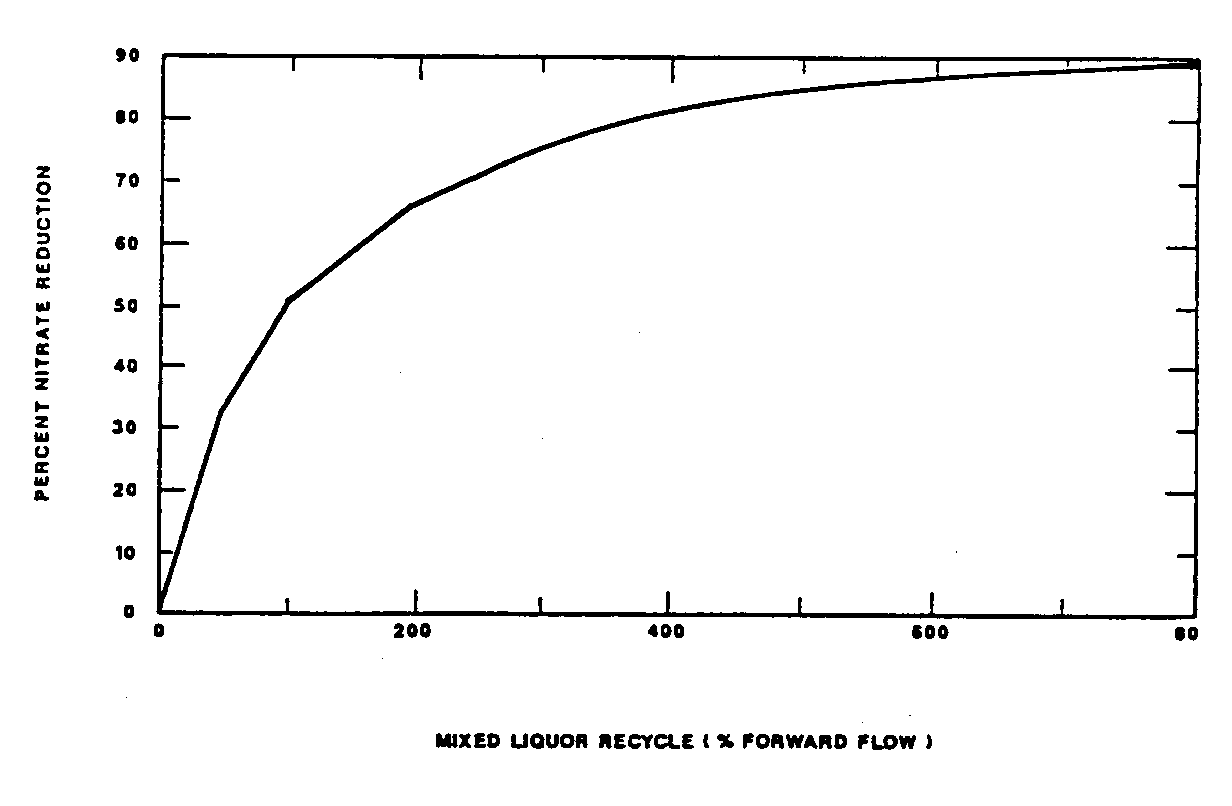 However, increased recycle rates lead to diminishing returns, that is the improvement in the denitrification potential is reduced as the recycle rate is further increased. This affect can be predicted mathematically and occurs because the mass of nitrate which is recycled is a function of both the first aerobic zone effluent nitrate concentration and the recycle flow rate. An increase in the recycle flow rate leads to an increase in the recycled mass, but at a diminishing rate because the nitrate-nitrogen concentration in the effluent from the first aerobic zone is decreased (due to increased denitrification). The following equation can be used to calculate the denitrification potential as a function of the mixed liquor recycle rate (expressed as a percentage of the process influent flow rate, R).
However, increased recycle rates lead to diminishing returns, that is the improvement in the denitrification potential is reduced as the recycle rate is further increased. This affect can be predicted mathematically and occurs because the mass of nitrate which is recycled is a function of both the first aerobic zone effluent nitrate concentration and the recycle flow rate. An increase in the recycle flow rate leads to an increase in the recycled mass, but at a diminishing rate because the nitrate-nitrogen concentration in the effluent from the first aerobic zone is decreased (due to increased denitrification). The following equation can be used to calculate the denitrification potential as a function of the mixed liquor recycle rate (expressed as a percentage of the process influent flow rate, R).
%Denite = [R/(1+R)] * 100 (3)
Several approaches are available to estimate the specific rate of denitrification in both the first and second anoxic zone. Empirical correlations can be used to estimate the specific rate of denitrification in first and second anoxic zones if information is not available on the biodegradability of the subject wastewater. A frequently used correlation is that developed by Burdick, Stensel, and Refling (1982). For the first anoxic zone, the specific rate of denitrification (SRND1, expressed in units of lb NO-N/lb mixed liquor suspended solids [MLSS]-day) is as follows:
SRDN1 = 0.03 F/M1 + 0.029 (4)
where F/M1 is the food-to-microorganism ratio for the first anoxic zone calculated as the mass of organic matter applied to the first anoxic zone (expressed in lb BOD5/day) divided by the activated sludge inventory in the first anoxic zone (expressed in lb). The following expression is used to calculate the specific rate of denitrification in the second anoxic zone (SRDN2, expressed in lb No-n/lb MLSS-day):
SRDN2 = 0.12 (MCRT)-0.706 (5)
where MCRT is the total MCRT in the process, including both the aerobic and anoxic zones.
When a detailed wastewater characterization of the wastewater is available, sophisticated process models such as the model developed by the South Africa Water Research Commission (Water Research Commission, 1984) or the International Association on Water Research and Control) Activated Sludge Model Number 1 (Henze, et al., 1987). These models have proven to accurately characterize the performance of full-scale biological nitrogen removal facilities when properly calibrated. The reader is referred to the recent U.S. EPA Nitrogen Control process design manual for more details on the use of these models (U.S. EPA, 1993).
Other Systems
While Figure 3 presents a prototype biological removal process, a large number of other process options are available. In those instances where a somewhat lesser degree of nitrogen removal is acceptable, a system consisting of only the first anoxic and aerobic zone and the mixed liquor recycle stream (as illustrated in Figure 5) may be adequate.

Other systems obtain nitrogen removal through the phenomenon known as simultaneous nitrification/denitrification. Nitrification and denitrification do not actually occur at the same time since different environmental conditions are required for the two biological reactions (as discussed above). However, nitrification and denitrification can occur in different portions of an aerated basin. This occurs by two mechanisms. First, both aerobic and anoxic zones can exist within an aerated basin due to the non-uniform conditions which exist in full-scale aeration basins. Second, anoxic zones can develop inside activated sludge flocs, even in aerated systems, due to the resistance to mass transfer which occurs as oxygen diffused through the floc. Both of these mechanisms operate to some extent in any real world aeration basin. Many systems exist which exploit this feature to encourage an extensive degree of nitrification and denitrification within the system. In many of these systems, the aeration rate must be carefully controlled so that the oxygen transferred just equals that needed to oxidize the carbonaceous organic matter and to nitrify the applied ammonia-nitrogen loadings, including the credit for denitrification. Table 5 summarizes several biological nitrogen removal systems.
Table 5
Other Example Biological Nitrogen Removal Process
* Oxidation Ditch
* Sequencing Batch Reactor
* Intermittant (Cyclic) Aeration Systems
* Schreiber Process
* Non-Uniform Aeration
Phosphorus Removal
Phosphorus removal occurs in a biological system by the accumulation of phosphorus in the process mixed liquor and increased wasting of the accumulated phosphorus in the waste activated sludge (WAS). Increased accumulation of phosphorus occurs due to the selection of high phosphorus content microorganisms. The phosphorus content of these microorganisms can approach 35 percent on a phosphorus to volatile suspended solids (VSS) basis (Wentzel, 1988).
Depending on the proportion of these microorganisms in the process mixed liquor, the phosphorus content of the activated sludge mixed liquor can be increased from a typical value of about two percent (P/VSS) for conventional activated sludge systems up to values that are typically in the 6 to 8 percent range and as high as 14 percent or more for biological phosphorus removal systems. Phosphorus I s a conservative substance, that is the mass of phosphorus in the process influent must equal the mass of phosphorus in the process effluents (both the liquid effluent and the WAS). In a biological phosphorus removal system, increased removal of phosphorus from the process influent is accomplished by increasing the mass of phosphorus in the WAS. Then, due to conservation of mass, a lower mass (and therefore, concentration) of phosphorus will be present in the process effluent. In other words, phosphorus removal in a biological phosphorus removal system occurs primarily due to mass balance principles.
Organism Selection Mechanism
What factors are responsible for the growth of these high phosphorus content microorganisms in biological phosphorus removal systems? They are selected by cycling the system mixed liquor between anaerobic and aerobic environments. To understand this statement, it is first necessary to define the term anaerobic.
As typically used in the description of biological nutrient removal systems, anaerobic is defined as the absence of either oxygen or nitrate-nitrogen as a terminal electron acceptor. Under these conditions, the capability of heterotrophic microorganisms to metabolize organic matter is dramatically reduced. Since no terminal electron acceptor is available, organic matter cannot be oxidized to generate energy. Fermentative reactions (the oxidation of an organic compound by another) can occur, but these reactions result in only limited production of energy for growth and other purposes by the microorganisms. In contrast, the phosphorus accumulating microorganisms are able to transport soluble organic matter across the cell membrane and store it under anaerobic conditions. They are able to this using energy stored in the form of high energy phosphate-to-phosphate bonds.
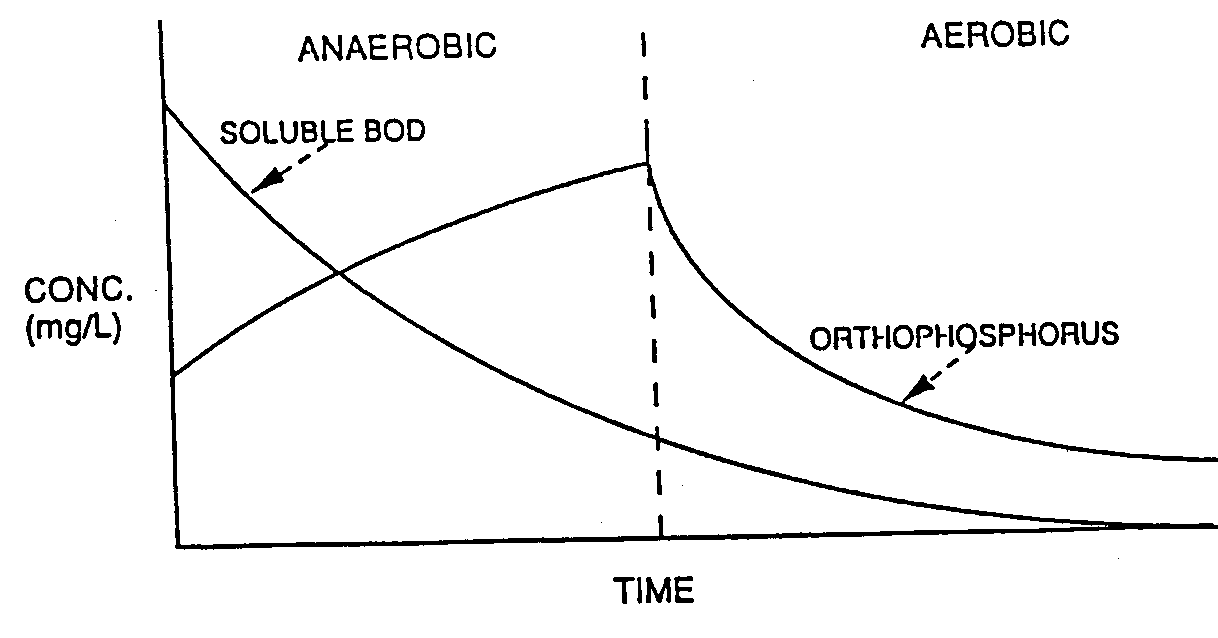 The biological phosphorus removing microorganisms contain high concentrations of polyphosphate, a phosphate polymer in which individual phosphate molecules are linked by high energy bonds. The phosphate which is generated as a result of the cleavage of the high energy phosphate-to-phosphate bonds diffuses out of the cell, resulting in an increase in soluble phosphate concentration. The release of phosphate and concurrent uptake of organic material is illustrated in Figure 6. However, as also illustrated in Figure 6, when the phosphorus accumulating microorganisms subsequently pass into the aerobic zone where oxygen is provided to allow aerobic metabolism, they oxidize the stored organic matter and generate energy which is used to take up phosphate from solution and store is as polyphosphate.
The biological phosphorus removing microorganisms contain high concentrations of polyphosphate, a phosphate polymer in which individual phosphate molecules are linked by high energy bonds. The phosphate which is generated as a result of the cleavage of the high energy phosphate-to-phosphate bonds diffuses out of the cell, resulting in an increase in soluble phosphate concentration. The release of phosphate and concurrent uptake of organic material is illustrated in Figure 6. However, as also illustrated in Figure 6, when the phosphorus accumulating microorganisms subsequently pass into the aerobic zone where oxygen is provided to allow aerobic metabolism, they oxidize the stored organic matter and generate energy which is used to take up phosphate from solution and store is as polyphosphate.
Polyphosphate is the biological phosphorus removing microorganisms has ben characterized as a "battery" which stores chemical energy for use as needed. The "battery" is discharged in the anaerobic zone to provide the energy necessary to transport and store soluble organic matter. It is then "recharged" in the aerobic zone as the stored organic matter is oxidized to generate the necessary energy. This characterization, although simplified, is accurate. Note also that the stored polyphosphate is responsible for the high phosphorus content of the phosphorus removing microorganisms. Consequently, any factor which encourages the metabolic pattern described above also improves the phosphorus removal capability of the process.
In summary, the mechanism responsible for the selection of the high phosphorus content microorganisms in a biological phosphorus removal process is cycling of the microorganisms between anaerobic and aerobic environments. The process influent wastewater is added to the anaerobic zone. Since the phosphorus removing microorganisms are able to take up and store the soluble organic matter contained in the influent wastewater while other heterotrophic microorganisms are not, the phosphorus removing microorganisms are placed at a competitive advantage and the population is enriched in these microorganisms. Because of the high phosphorus content of these microorganisms, the phosphorus content of the mixed liquor is increased. As a consequence, the mass of phosphorus contained in the WAS removed from the process is increased, resulting in a reduced mass of phosphorus in the process effluent.
Prototype System
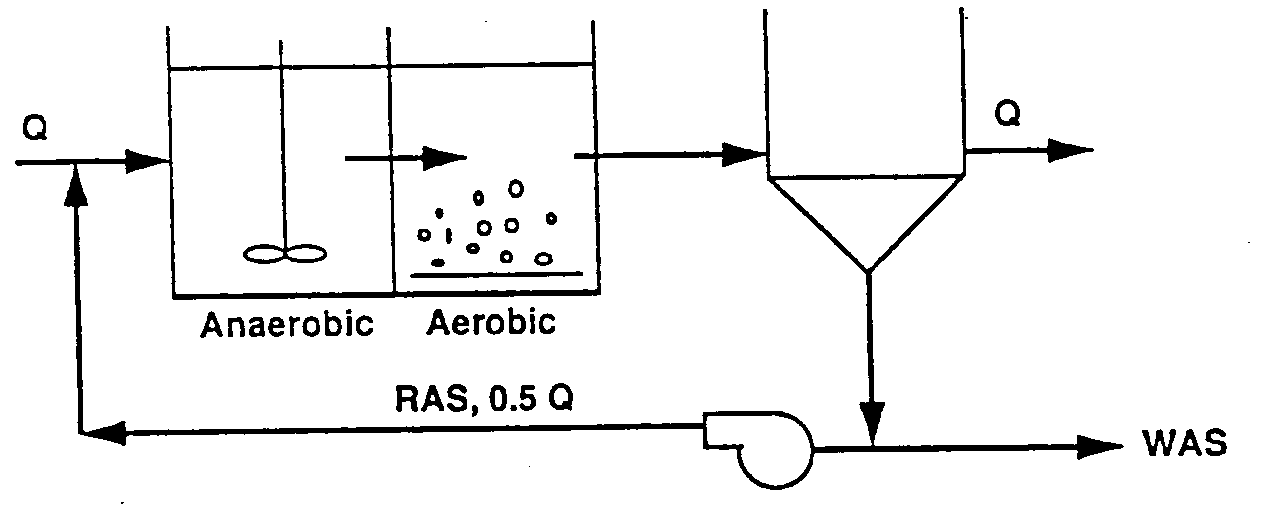 Figure 7 illustrates the basic configuration of a biological phosphorus removal system. It consists of an initial anaerobic zone which receives return activated sludge (RAS) from the clarifier and the process influent wastewater. The uptake of soluble organic matter occurs in this zone, along with the corresponding release of phosphate. The mixed liquor then flows out of the anaerobic zone and into the aerobic zone where organic matter is oxidized and phosphate uptake occurs. The phosphorus removing microorganisms grow relatively slowly (but generally faster than the nitrifying bacteria). Both laboratory and full-scale experience indicate that a process MCRT on the order of 3 days must be maintained to avoid wash-out of the phosphorus removing bacteria (Mamais and Jenkins, 1992). Typically the hydraulic residence time in the anaerobic zone is 0.75 to 1.0 hour based on the process influent flow rate. Factors which affect the size of the anaerobic zone are discussed below.
Figure 7 illustrates the basic configuration of a biological phosphorus removal system. It consists of an initial anaerobic zone which receives return activated sludge (RAS) from the clarifier and the process influent wastewater. The uptake of soluble organic matter occurs in this zone, along with the corresponding release of phosphate. The mixed liquor then flows out of the anaerobic zone and into the aerobic zone where organic matter is oxidized and phosphate uptake occurs. The phosphorus removing microorganisms grow relatively slowly (but generally faster than the nitrifying bacteria). Both laboratory and full-scale experience indicate that a process MCRT on the order of 3 days must be maintained to avoid wash-out of the phosphorus removing bacteria (Mamais and Jenkins, 1992). Typically the hydraulic residence time in the anaerobic zone is 0.75 to 1.0 hour based on the process influent flow rate. Factors which affect the size of the anaerobic zone are discussed below.
The process configuration presented in Figure 7 is the simplest biological phosphorus removal process, and it is quite appropriate and effective for wastewater treatment applications where nitrification is not required or desired. However, in such a configuration biological phosphorus removal is adversely affected if the aerobic zone is large enough to allow nitrifying bacteria to grow. This adverse impact occurs because nitrification in the aerobic zone results in the production of nitrate-nitrogen which is recycled to the anaerobic zone in the RAS flow stream. Thus recycle of nitrate-nitrogen provides a terminal electron acceptor in the initial mixed zone that allows heterotrophic denitrifying bacteria to compete with the phosphorus removing bacteria for organic matter contained in the influent wastewater. Under these circumstances, a reduced competitive advantage is provided for the phosphorus removing bacteria, resulting in reduced enrichment of the population with high phosphorus content microorganisms and reduced biological phosphorus removal capability. As a consequence, a variety of process configurations have been developed which restrict the recycle of nitrate-nitrogen to the anaerobic zone in nitrifying biological phosphorus removal systems.
Other Systems
One such system is the VIP process (VIP stands for the Virginia Initiative Plant) illustrated in Figure 8. This process consists of an initial anaerobic zone to select for high phosphorus content microorganisms, an anoxic zone with mixed liquor recycle to accomplish denitrification (resulting in total nitrogen removal), and an aerobic zone for nitrification and phosphorus uptake. In this process configuration, RAS (which will contain nitrate-nitrogen) is directed to the anoxic zone where denitrification is intended to occur. An additional in-process recycle stream is introduced in the form of the anoxic recycle (ARCY). This recycle stream takes denitrified mixed liquor exiting the anoxic zone and delivers it to the process influent as it flows into the anaerobic zone. This recycle stream is necessary to deliver mixed liquor into the anaerobic zone to allow biological reactions to occur. Because a denitrified mixed liquor is recycled, however, nitrate-nitrogen addition to the anaerobic zone is minimized, thereby minimizing the interference of nitrate-nitrogen with biological phosphorus removal and maximizing the biological phosphorus removal capability of the process.

As discussed above, the nature of the organic matter added to the biological nutrient removal process affects nitrogen removal. This is also true for biological phosphorus removal. This occurs because of the highly specialized requirements of the phosphorus accumulating microorganisms required to accomplish biological phosphorus removal.
As discussed above, in the anaerobic zone the phosphorus accumulating microorganisms transport soluble organic matter across the cell membrane and store it as intracellular carbon storage products. In fact, these mircoorganisms will generally only transport and store low molecular weight volatile fatty acids (VFAs) such as acetic and propionic acid (Randall, Barnard, and Stensel, 1982). Consequently, the mass of VFAs available to these microorganisms dramatically affects the performance of biological phosphorus removal systems. Some wastewaters have sufficient concentrations of VRAS present, in many cases due to fermentative reactions which occur as the wastewater is transported to the wastewater treatment plant in the collection system. In many instances, however, this is not the case. In such instances, biodegradable organic matter must be fermented to generate the necessary VFAs. Several approaches are available to generate the VFAs necessary to accomplish biological phosphorus removal. The two which will be discussed here are fermentation or readily biodegradable organic matter in the anaerobic zone of the biological phosphorus removal process and the fermentation of particulate matter in a separate fermenter.
In any biological phosphorus removal process, a portion of the readily biodegradable organic matter (as defined and described above under the discussion of biological nitrogen removal) will be fermented in the anaerobic zone to produce VFAs. This occurs because many of the heterotrophic microorganisms in biological wastewater treatment systems have fermentative capabilities. By definition, the readily biodegradable organic matter is available to the microorganisms for biological reaction. In an anaerobic environment, where the readily biodegradable organic matter cannot be oxidized due to the absence of a terminal electron acceptor, it will be fermented to produce VFAs and other fermentation products.
In general, the fermentation process is relatively slow, even when readily biodegradable organic matter is being fermented. Fundamental parameters are not yet available to characterize the reactions occurring in the anaerobic zone. However, experience indicates that different anaerobic zone hydraulic residence times (HRT, computed based on the process influent flow rate) can be used to characterize the reactions occurring in the anaerobic zone. For example, experience indicates that the uptake of VFAs in the anaerobic zone is a very rapid reaction as indicated by the fact that it is typically fully complete in an anaerobic zone HRT of 0.75 hour or less. The fermentation of readily biodegradable organic matter is a slower process, generally requiring an anaerobic zone HRT of 1 to 2 hours or more. In fact, this difference in required reaction time provides guidance as to the required anaerobic zone HRT for a particular application. If the influent wastewater contains significant concentrations of pre-formed VFAs, then a relatively short anaerobic zone HRT can be used. If, on the other hand, significant fermentation is required in the anaerobic zone to generate VFAs, then a longer anaerobic zone HRT will be required.
Also note that the recycle of nitrate-nitrogen to the anaerobic zone will interfere with fermentation reactions. Given the option of either using nitrate-nitrogen to oxidize readily biodegradable organic matter or of fermenting the readily biodegradable organic matter, oxidation using nitrate-nitrogen will dominate due to the greater amount of energy which the microorganisms can obtain through oxidative reactions. This is another reason that the recycle of nitrate-nitrogen to the anaerobic zone can interfere with effective and efficient biological phosphorus removal.
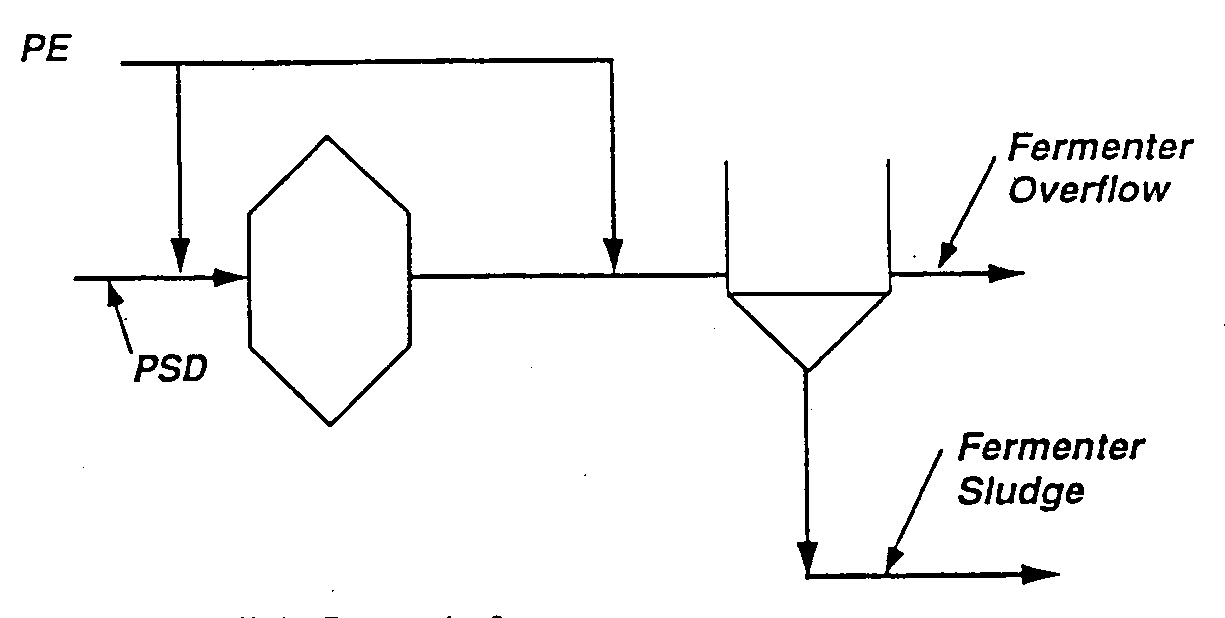
The fermentation of particulate biodegradable organic matter is a relatively slow reaction which must generally be accomplished external to the biological nutrient removal process. Figure 9 illustrates a typical approach which involves the addition of primary sludge (PSD) to a mixed reactor where the particulate matter is fermented to generate VFAs (Skalsky, et al., 1992). This reaction generally takes 2 to 3 days (not hours). The fermented primary sludge is then sent to a liquid/solids separation device such as a gravity thickener. In some instances an elutriation flow such as primary effluent (PE) is added to "wash" the soluble VFAs from the remaining particulate matter. The overflow which contains high concentrations of VFAs is directed to the anaerobic zone of the biological nutrient removal process, while the underflow contained the remaining particulate matter is sent to sludge treatment. Several primary sludge fermentation configurations are available and are used in full-scale applications.
A wide variety of biological phosphorus or biological nitrogen and phosphorus removal process options are available, each with their own advantages and disadvantages. Table 6 summarizes several of these for reference by the reader. These processes are described in detail in the references discussed below.
Table 6
Other Example Biological Phosphorus Removal Process
* A2/O
* Five-stage Bardenpho
* University of Capetown (UCT)
* VIP
* OWASA
BIOLOGICAL NUTRIENT REMOVAL PERFORMANCE
 Approaches for sizing the various components of a biological nutrient removal systems were briefly reviewed in the previous section of this paper and are discussed in more detail in the references to be presented below. This section provides summary information on the performance capabilities of biological nutrient removal systems.
Approaches for sizing the various components of a biological nutrient removal systems were briefly reviewed in the previous section of this paper and are discussed in more detail in the references to be presented below. This section provides summary information on the performance capabilities of biological nutrient removal systems.
Biological Nitrogen Removal
Studies indicate that the performance of properly sized biological nitrogen removal systems is stable and predictable. The predictability of biological nitrogen removal systems is illustrated in Figure 10 where a probability plot of monthly average effluent total nitrogen concentrations from a variety of full-scale and large pilot-scale biological nutrient removal facilities is presented. The results generally fall into two bands. The upper band corresponds to facilities with a single anoxic zone, such as illustrated in Figure 5. The influent total nitrogen concentration for many of these facilities was in the 30 to 40 mg-N/L range. As indicated in Figure 10, these facilities were able to reduce the total nitrogen concentration into the 6 to 8 mg-N/L range on average. In fact, effluent total nitrogen concentrations were seldom above 10 mg-N/L (with the exception of the Fayetteville, AR A/O facility which does not incorporate mixed liquor recycle). As discussed above, total nitrogen removal in single anoxic zone facilities is limited by the fact that nitrate-nitrogen must be recycled from the downstream aerobic zone to the upstream anoxic zone. As a consequence, nitrate-nitrogen concentrations cannot be reduced to near-zero levels. However, as indicated in Figure 10, a high degree of nitrogen removal can be achieved with such systems.
The second performance band indicated in Figure 10 is for facilities with two anoxic zones, as described in Figure 3. A higher degree of nitrogen removal (i.e. lower effluent total nitrogen concentrations) would be expected for such processes and is observed in Figure 10. Average effluent total nitrogen concentrations are about 2 mg-N/L, and they are reliably below 3 and 4 mg-N/L. In these cases, almost complete removal of inorganic nitrogen (ammonia, nitrate, nitrite) is being achieved. The remainder of the effluent total nitrogen is non-biodegradable
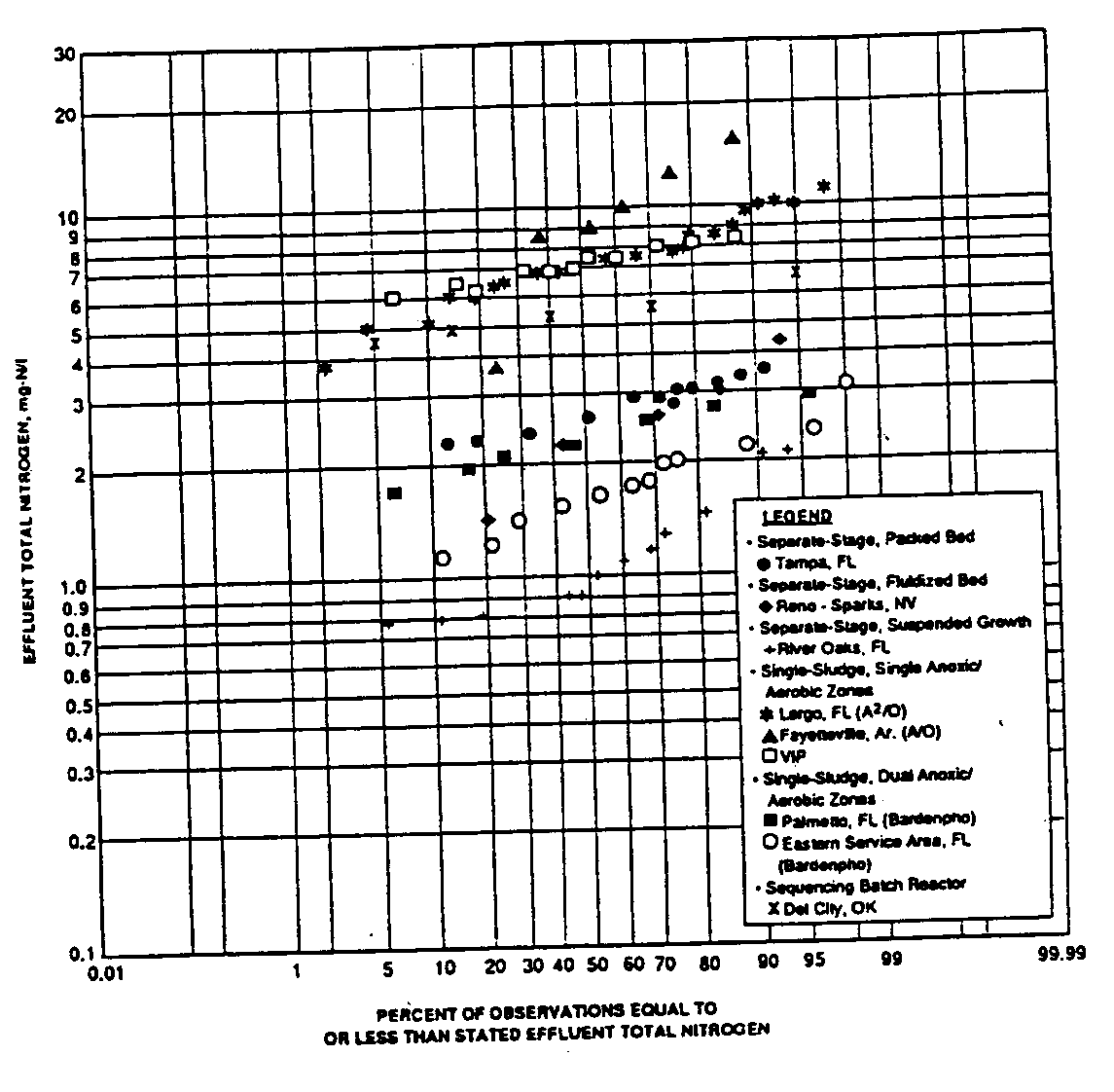 organic nitrogen, present either in particulate or soluble forms. Municipal wastewaters contain a certain amount of non-biodegradable organic nitrogen, and some non-biodegradable organic nitrogen is produced as a by-product of the biodegradation of organic matter. As a consequence, it is not generally possible to produce an effluent from a biological nitrogen removal process with zero nitrogen. The concentration of non-biodegradable soluble organic nitrogen in a biological process effluent is often in the 1 to 2 mg-N/L range. This is reflected in the performance data presented in Figure 10.
organic nitrogen, present either in particulate or soluble forms. Municipal wastewaters contain a certain amount of non-biodegradable organic nitrogen, and some non-biodegradable organic nitrogen is produced as a by-product of the biodegradation of organic matter. As a consequence, it is not generally possible to produce an effluent from a biological nitrogen removal process with zero nitrogen. The concentration of non-biodegradable soluble organic nitrogen in a biological process effluent is often in the 1 to 2 mg-N/L range. This is reflected in the performance data presented in Figure 10.
Experience also indicates that biological nitrogen removal processes will many times perform better than their original design assumptions. This occurs due to the occurrence of simultaneous nitrification/denitrification in the aerobic zones, as discussed above. Simultaneous nitrification/denitrification varies with the type of oxygen transfer system in the aerobic zone and with the operational procedures. It can be encouraged by restricting and controlling oxygen input. The extent to which simultaneous nitrification/denitrification occurs cannot be precisely predicted at this time and is a matter of experience. However, it is comforting that most biological nitrogen removal processes will generally perform as well or better than predicted based on their designs.
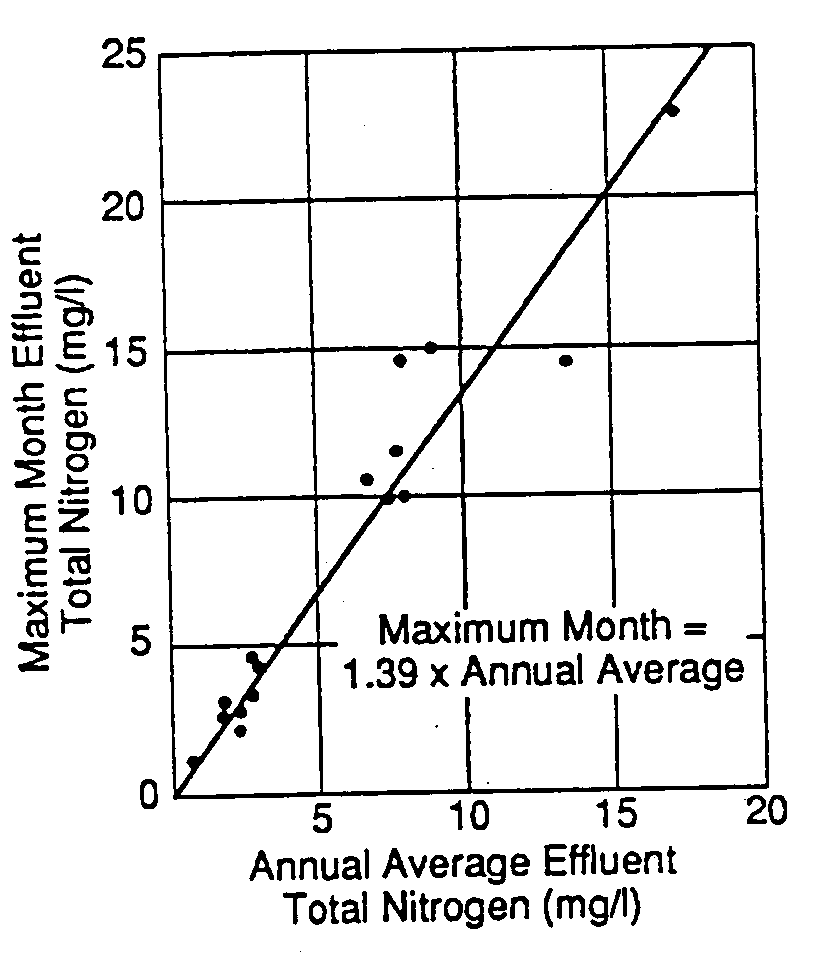
The stability of biological nitrogen removal processes is illustrated by Figure 11 which presents the relationship between annual average and highest monthly average effluent total nitrogen concentrations for a variety of biological nitrogen removal facilities. These results indicate that, in the worst month, effluent total nitrogen concentrations from biological nitrogen removal facilities generally be about 40 percent higher than the long-term average value from the same facility. In comparison, similar studies of wastewater treatment plants practicing BOD5 and TSS removal indicate that the highest month effluent BOD5 and TSS concentrations may be 50 percent or more higher than the long-term average. This comparison suggests a higher degree of reliability for biological nitrogen removal than for BOD5 and TSS removal (Morales, Daigger, and Borberg, 1991).
 Biological Phosphorus Removal
Biological Phosphorus Removal
Experience with biological phosphorus removal systems indicates a higher degree of performance variability than for biological nitrogen removal facilities. Historically, this has resulted to some extent because the mechanisms responsible for biological phosphorus removal were not as well understood as those responsible for biological nitrogen removal. Some evidence also suggests that the biological phosphorus removal process may be inherently more variable.
Analyses of the effluent quality from biological phosphorus removal facilities similar to those conducted for biological nitrogen removal facilities (and illustrated in Figure 10) have been conducted (Morales, Daigger, and Borberg, 1991; Sedlak, 1991). These analyses have indicated a more viable performance pattern with a greater number of parameters affecting process effluent total phosphorus concentrations. Subsequent analyses have indicated that the performance of biological phosphorus removal processes is best characterized by the amount of phosphorus removed, not by the effluent total phosphorus concentration. This occurs because of the phosphorus removal mechanism operating in a biological phosphorus removal system; phosphorus is accumulated in the mixed liquor and subsequently removed with the waste sludge. Recognition of the operative mechanism in a biological phosphorus removal process led to the concept of the BOD5 to Phosphorus Removal ratio as a means for characterizing biological phosphorus removal process performance (Daigger, et. al., 1990; Sedlak, 1991).
This parameter recognizes the fundamental phosphorus removal mechanism operating in a biological phosphorus removal system and the fact that phosphorus removal can be limited by the mass of organic matter available to allow the necessary high phosphorus content microorganisms to grow.
The BOD5 to Phosphorus Removal ratio is the ratio of the BOD5 applied to the process divided by the phosphate removed by the process. For an operating process, it is calculated as follows:
(6)
where BOD5i is the concentration of BOD5 in the phosphorus removal process influent, TPi is the concentration of total phosphorus in the phosphorus removal process influent, and SPe is the concentration of soluble phosphate in the process effluent. Use of the BOD5 to Phosphorus Removal ratio requires distinction between a biological phosphorus removal process which is phosphorus limited and one which is BOD5 limited.
In a phosphorus limited process, more than sufficient BOD5 is present in the process effluent to remove essentially all of the influent phosphorus. In such a situation, more phosphorus could be removed if it were available and, consequently, the effluent soluble phosphate concentration is relatively low (say, 0.5 mg-P/L or lower). This is a desirable situation if a high level of performance (i.e. low effluent phosphorus concentrations) is required. For a BOD5 limited process, on the other hand, the removal of phosphorus is limited by the amount of BOD5 available. In such situations, the concentration of soluble phosphorus in the process effluent will be relatively high, generally greater than about 1 mg-P/L.
Experience also indicates that the BOD5 to Phosphorus Removal ratio for various biological phosphorus removal facilities varies, depending on the efficiency of the biological phosphorus removal process and the characteristics of the wastewater. In fact, the BOD5-to-Delta-Phosphate ratio achieved by various facilities when operating under BOD5 limited conditions indicates the efficiency of the system.
Table 7 (given on the following page) provides some general indication of the efficiency of various often used biological phosphorus removal processes. Data such as these can be used to estimate the performance that can be achieved using various process options in a particular application.
Table 7
Example TBOD5 to Phosphorus Removal Ratios
TBOD5/Phosphorus Removal
Process (mg/mg)
A/O, A2/O
Non-nitrifying 15 to 20
Nitrifying 20 to 25
VIP 15 to 20
Bardenpho 25 to 30
Several other factors affect the performance of wastewater treatment plants using biological phosphorus removal processes, as illustrated in Table 8. One is the method of sludge handling used at the facility. As discussed above, the phosphorus accumulated in the mixed liquor of a biological phosphorus removal process can be released into solution quite easily if the mixed liquor is exposed to readily biodegradable organic matter under anaerobic conditions. If such release occurs as the waste sludge is being processed and if the sludge is subsequently thickened or dewatered, the removed phosphorus will be separated from the concentrated solids. If the liquid stream from the liquid-solids separation process is subsequently recycled to the liquid process train at the wastewater treatment process, much of the biologically removed phosphorus will likewise be returned to the liquid treatment process. If too much phosphorus is recycled back to the liquid treatment process, the phosphorus removal capability of the biological phosphorus removal process will be overloaded, leading to a deterioration in effluent quality. Consequently, the fate of the removed phosphorus must be carefully controlled as the waste biological sludge is processed to avoid the release and recycle of that removed phosphorus.
Table 8
Factors Affecting Biological Phosphorus Removal
Wastewater Configuration
* BOD5/TP * Zone Size and Loading
* Biodegradability * Nitrate Recycle
* VFA Content * Aeration System
* Temperature * Configuration and Operation
* Inert Content * Effluent TSS
* Variability in Characteristics * Approach to Fermentation
* Sludge Handling Process and Recycle
A second factor which can dramatically impact the performance of a biological phosphorus removal facility is the concentration of suspended solids in the process effluent. This occurs because, as phosphorus is accumulated in the process mixed liquor, the proportion of phosphorus in the mixed liquor suspended solids increases. As a consequence, any suspended solid present in the process effluent can contribute significant concentrations of phosphorus. Fortunately, both research and experience indicates that the concentration of suspended solids in biological phosphorus removal process effluents is often relatively low (Morales, Daigger and Borberg, 1991). However, the potential adverse impact of high effluent suspended solids concentrations on biological phosphorus removal process performance must be recognized.
AVAILABLE RESOURCES
A number of good resources are available to assist those interested in learning more about biological nutrient removal systems. Table 9 provides a list of key references that the beginner will find useful in gaining an overall perspective on biological nutrient removal processes. The individual references contained within these key references can provide the more advanced user with the detailed information needed to provide a more in-depth understanding of BNR systems. Knowledge concerning the design and operation of BNR systems is advancing rapidly. Consequently, the interested user is cautioned to review the current literature to follow the introduction and evaluation of new BNR system concepts.
TABLE 9
Key References
* Phosphorus and Nitrogen Removal from Municipal wastewater: Principles
And Practice, 1991.
* Process Design Manual for Nitrogen Control, 1993.
* Theory, Design and Operation of Nutrient Removal Activated Sludge Processes,
1984.
* Nutrient Control, WPCF, 1983.
* Design and Retrofit of Wastewater Treatment Plants for Biological Nutrient
Removal, 1992.
REFERENCES
Burdick, C.R., D.R. Refling, and H.D. Stensel (1982), "Advanced Biological Treatment to Achieve Nutrient Removal," Jour. Water Pollut. Control Fed., 54, 1078.
Daigger, G.T., L.M. Morales, J.R. Borbert, and G.D. Waltrip (1990), "Full-Scale and Pilot-Scale Experience with the VIP Process," Presented at the First Australian Conference on Biological Nutrient Removal (BNR1), Bendigo, Australia.
Grady, C.P.L., Jr. and H.C. Lim (1980), Biological Wastewater Treatment: Theory and Applications, Marcel Dekker, Inc., New York.
Henze, M., et al. (1987), "A General Model for Single Sludge Wastewater Systems," Water Res., 21, 505.
Mamais, D. And D. Jenkins (1992), "The Effects of MCRT and Temperature on Enhanced Biological Phosphorus Removal," Water Sci. Tech., 26:4/5, 955.
Morales, L.M., G.T. Daigger, and J.R. Borbert (1991), "Capability Assessment of Biological Nutrient Removal Facilities," Res. Jour. Water Pollut. Control Fed., 63, 900.
National Research Council (1993) Managing Wastewater in Coastal Urban Areas, National Academy Press, Washington, D.C.
Randall, C.W., J.L. Barnard, and H.D. Stensel (1992), Design and Retrofit of Wastewater Treatment Plants for Biological Nutrient Removal, Technomic Publishing Co., Inc., Lancaster, Pennsylvania.
Sedlak, R.I., Editor (1991), Phosphorus and Nitrogen Removal from Municipal Wastewater: Principles and Practice, Second Edition, Lewis Publishers, Boca Raton, Florida.
Skalsky, D.S., et al., (1992), "Fermentation of Primary Sludge for Volatile Acid Production," Liquid Treatment Processes Symposium, Proceedings of the 65th Annual Conference and Exposition, Water Environment Federation, Alexandria, Virginia, 331.
U. S. EPA (1993), Process Design Manual for Nitrogen Control, EPA/625/R-93/010, Washington, D. C.
Water Pollution Control Federation (1983), Nutrient Control, Manual of Practice FD-7, Alexandria, Virginia.
Water Research Commission (1984), Theory, Design and Operation of Nutrient Removal Activated Sludge Processes, Pretoria, South Africa.
Wentzel, M.C., et al., (1988), "Enhanced Polyphosphate Organism Cultures in Activated Sludge Systems," Water SA, 14, 81.